
सीएसआईआर-राष्ट्रीय भौतिक प्रयोगशाला
CSIR-National Physical Laboratory

Biomedical Metrology
About
- Development, operation and maintenance of biomedical equipment standards
- Synthesis and Characterization of Electroactive materials for Biosensing applications
- Research and Development on “Dynamic studies of electroactive materials for Biomolecular Recognition in Miniaturized system”
- Providing calibration services through dissemination of traceability of physical parameters to accredited testing laboratories and other stakeholders in healthcare sector
- Promoting and Strengthening quality system in the country through conducting time-to-time awareness program, workshop and hand on trainings etc. among various stakeholders
Research Areas
- Synthesis and characterization of novel electroactive materials like inorganic/organic hybrid materials including electro-optic liquid crystals, conducting polymers, dendrimers, hierarchical structures, nanomaterials, quantum dots etc. for the biomolecular sensing.
- Establishing structure-property relations for biomolecular transduction using electrochemical, low ac frequency impedance, optical, opto-electronic, and electro-optic studies
- Design and fabrication of highly sensitive bio-platform for the detection of biomarkers for chronic diseases, water and food borne infections
Metrology Activities
Biomedical metrology group CSIR-NPL is working towards establishment and realization of National Standards for biomedical metrology. The group is actively engaged in the design, development and dissemination of biomedical equipment standards compatible to International standards for biomedical instrumentation. Group has successfully completed numerous sponsored/ inhouse projects in the field of Biomedical Instrumentation/ Metrology.
Calibration Capability of Defibrillator and Defibrillator Analyser
Group has established an apex level calibration facility for defibrillator analyzer by installing a set-up of a primary standard of defibrillator. This NPLI facility is the “first-of-its kind” in India to cater the needs of quality assurance in the area of healthcare. Biomedical Metrology has been working in establishing biomedical equipment standards as per the New Medical device rules and policies. In this regard, we have established an apex level calibration facility for defibrillator analyzer by installing a set-up of a primary standard of defibrillator with its physical parameters traceable to national standards (Schematic shown in Fig. 1). Defibrillator works by supply monobasic /biphasic pulse to patient with energy levels in range of 50J to 360J. This calibration facility is providing services to various stakeholders with effect from 1st September 2018.
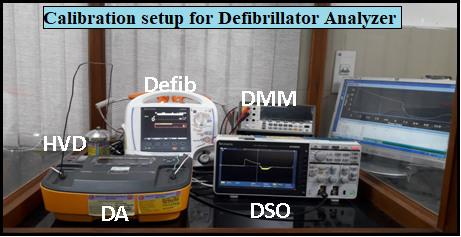
Fig.1: Schematic diagram of established calibration facility for
Defibrillator and defibrillator analyser
Calibration Facility for Infusion Pump Analyzer
Group has designed the calibration setup for infusion pump analyser as per IS 13450 (Part 2/Sec 24): 2009 equivalent to IEC 60601-2-24 describes a calibration procedure particularly for the medical electrical infusion pump device. The schematic of the designed infusion pump analyzer set up is shown in Fig. 2. In a typical calibration procedure of infusion pump analyzer, the volumetric flow rate is calculated by directly converting the mass into volume with respect to a reference temperature normally at 20o C , as per methodology mentioned in ISO/TR 20461 (Determination of uncertainty for volume measurements by gravimetric method). The calibration facility is expected to be completed for dissemination to stake holders and industries soon.

Fig. 2: Schematic of Infusion pump analyzer calibration set up at CSIR-NPL
Blood Penetration Setup
The group is setting up the testing of personal protective equipment, which included blood penetration test as per Indian standard (BIS 16546:2016. In the procedure, material used in PPE kits is placed in contact with pressurized synthetic blood, and the resistance offered by test specimen to synthetic blood at varying pressure is tested. A dedicated test setup as per ASTM 903 standard has been designed and fabricated.
Major Research Highlights
The Biomedical Metrology group carries out a wide range of frontier and applied research activities related to synthesis and characterization of novel electroactive materials such as quantum dots, dendrimers, organic and inorganic nanomaterials, conducting polymers and liquid crystals and thin films and exploitation of their extraordinary properties in the field of biomolecular recognition using various transduction principles such as electrochemical transduction, optical transduction and surface plasmonic resonance etc for biomedical applications, especially in the diagnosis of cardiovascular, food toxins and infectious diseases. The group has more than 100 publications in peer reviewed SCI journals and filed more than 5 patents in the last 10 years in the field of biomolecular electronics and biosensing applications. The Biomedical Metrology section have sophisticated instrumentation facilities such as Atomic Force Microscope, UV-Visible spectrophotometer, Electrochemical workstation and particle size analyzer, etc.
Team
Dr. Rajesh
Head
Biomedical Metrology Section & Chief Scientist
Email: rajesh@nplindia.org
- Dr. (Ms) Sumana Gajjala
Sr. Principal Scientist
Email: sumanag@nplindia.org - Dr. Ved Varun Agarwal
Sr. Principal Scientist
Email: vedvarun@nplindia.org - Ms. Sudesh Yadav
Scientist
Email: sudesh.yadav@nplindia.org - Mr. Arun Kant Singh
Scientist
Email: arunkant.singh@nplindia.org - Mr. Vinod Kumar Tanwar
Technical Officer-II
Email: vkt@nplindia.org - Ms. Vishesh
Technical Officer-II
Email: vishesh@nplindia.org - Mr. Manoj Kumar Pandey
Technical Officer-I
Email: mkpandey@nplindia.org - Dr. Vikash Sharma
Technical Officer-I
Email: vicky@nplindia.org
All Rights Reserved - The Official Website of CSIR-National Physical Laboratory, CSIR, under Ministry of S & T, Govt. of India
Site Designed & Managed by Knowledge Resource Centre
CSIR-NPL, New Delhi
India